INTRODUCTION
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, clonal hematopoietic stem cell disorder characterized by chronic complement-mediated intravascular and extravascular hemolysis (IVH and EVH), and thrombosis. C5 inhibitors reduce IVH by inhibiting membrane attack complex formation and are the current standard of treatment for PNH. EVH persists in the presence of C5 inhibition. Pegcetacoplan is a C3 inhibitor targeted to control both IVH and EVH. The PADDOCK and PALOMINO studies assessed the safety and preliminary efficacy of pegcetacoplan in complement inhibitor-naïve patients.
METHODS
PADDOCK was a phase 1b, open-label, pilot study (NCT02588833) with 2 cohorts. Subjects in cohort 1 received pegcetacoplan at a suboptimal dose of 180 mg/day for 28 days; subjects in cohort 2 received pegcetacoplan 270 mg/day for up to 1 year. Subjects could participate in both cohorts. PALOMINO was a phase 2a, open-label, single-cohort study (NCT03593200) in which subjects received pegcetacoplan 270 mg/day for up to 1 year. Both studies enrolled subjects ≥18 years old diagnosed with PNH and no prior treatment with eculizumab. Primary efficacy endpoints in both studies were change from baseline in lactate dehydrogenase (LDH), haptoglobin, and hemoglobin. Secondary efficacy endpoints included change from baseline in reticulocyte count, total bilirubin, and Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score. The number of packed red blood cell transfusions was also recorded. Primary safety endpoints were the incidence and severity of treatment emergent adverse events (TEAEs).
RESULTS
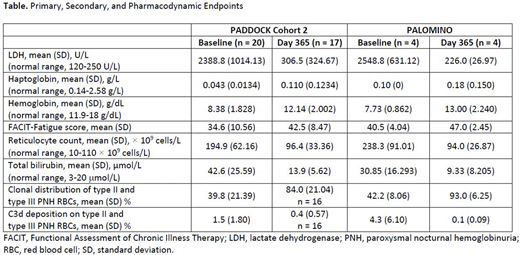
Three subjects were enrolled in PADDOCK cohort 1 and 20 in cohort 2; 1 subject was enrolled in both cohorts. PALOMINO enrolled 4 subjects. PADDOCK cohort 2 and PALOMINO efficacy data are shown in the Table. At baseline, mean LDH levels were >8× upper limit of normal in both studies. At day 365, mean LDH had decreased to <2× upper limit of normal in PADDOCK and was within the normal range in PALOMINO. At baseline, mean hemoglobin levels were below the lower limit of normal in both studies. At day 365, mean hemoglobin levels had increased to within the normal range in both studies. In PALOMINO, 3 subjects achieved hemoglobin levels above the lower limit of normal at day 365, and 1 subject had a hemoglobin level of 10.14 g/dL. An increase from baseline in mean FACIT-Fatigue scores of 7.1 and 6.5 at day 365 was observed in PADDOCK and PALOMINO, respectively. In PADDOCK and PALOMINO, mean reticulocyte count decreased from 194.9 × 109 cells/L and 238.3 × 109 cells/L at baseline, respectively, to 96.4 × 109 cells/L and 94.0 × 109 cells/L at day 365. Total bilirubin levels also decreased from 42.6 µmol/L and 30.85 µmol/L at baseline to 13.9 µmol/L and 9.33 µmol/L at day 365 in PADDOCK and PALOMINO, respectively. In PADDOCK cohort 2, 18/20 subjects received ≥1 transfusion in the 12 months prior to screening, and 13/20 subjects did not require a transfusion during the study. In PALOMINO, all subjects received ≥1 transfusion in the 12 months prior to screening, and none required a transfusion during the study. Overall, 143 TEAEs were reported in 19/22 subjects (86.4%) in PADDOCK cohorts 1 and 2, of which 35 were either possibly or probably related to pegcetacoplan. Thirteen serious adverse events (SAEs) were reported in PADDOCK. Three SAEs (aplastic anemia, abdominal neoplasm, and hypersensitivity) led to pegcetacoplan discontinuation in 3 subjects. Two of these SAEs (aplastic anemia and abdominal neoplasm) resulted in study discontinuation. The aplastic anemia SAE was fatal and considered not related to pegcetacoplan. In PALOMINO, 60 TEAEs were reported in 3 (75.0%) subjects, of which 52 were possibly treatment-related. One SAE (rib fracture) was reported and was considered not related to pegcetacoplan. In PALOMINO, no TEAEs led to death, pegcetacoplan discontinuation, or study discontinuation.
CONCLUSIONS
In PADDOCK and PALOMINO, pegcetacoplan improved hematological parameters by controlling both IVH and EVH in patients with PNH. Improvements in clinical parameters were also observed. Pegcetacoplan was shown to have an acceptable safety profile and was generally well tolerated in both studies. PADDOCK and PALOMINO demonstrate the therapeutic potential of pegcetacoplan in the treatment of PNH and support further evaluation in the ongoing phase 3 PRINCE trial (NCT04085601) in complement inhibitor-naïve patients.
Wong:Alexion: Consultancy, Honoraria, Research Funding, Speakers Bureau; Apellis: Research Funding, Speakers Bureau; Roche: Consultancy, Honoraria, Research Funding, Speakers Bureau. Sathar:Novo Nordisk: Honoraria; Bayer: Honoraria; Roche: Honoraria. Tse:MSD: Research Funding; Janssen: Research Funding. Roman:Alexion: Speakers Bureau; Novartis: Speakers Bureau. Amine:Acibadem City Clinic Tokuda Hospital: Current Employment. Tan:Apellis: Consultancy, Patents & Royalties. Di Casoli:Apellis: Current Employment, Current equity holder in private company. Deschatelets:Apellis: Current Employment, Current equity holder in private company, Patents & Royalties. Francois:Apellis: Current Employment, Current equity holder in private company, Patents & Royalties. Grossi:Apellis: Current Employment, Current equity holder in private company, Patents & Royalties. Bogdanovic:Takeda (regional office in Serbia, only local activities in Serbia): Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer (regional office in Serbia, only local activities in Serbia): Membership on an entity's Board of Directors or advisory committees; Apellis: Other: Investigator fee in clinical trial; Novartis Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Pegcetacoplan is an investigational drug for the treatment of paroxysmal nocturnal hemoglobinuria
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal